Agreements
UK-CONFAP Call for Health Systems Research Networks
Application and Case for Support Guidance
Contents
1. Important application information
2. Application process
2.1 Application and review process
2.2 UK applicants
2.3 Brazilian applicants
2.4 Page lengths 3. Additional document information
3.1 Case for support
3.2 Pathways to impact 4. Budget
5. Joint electronic System (Je-S)
5.1 Creating a Je-S account
5.2 Guidance for overseas organisations to be registered on J-S 6. Assessment process and criteria
7. Agreements 7.1 Collaboration agreement
7.2 Intellectual property
7.3 Material transfer agreements
7.4 Ethics
7.5 Humans / human tissue 8. Terms and conditions
Annex 1
1. Important application information (índice)
This initiative will provide funding for collaborative research projects. Researchers will be responsible for developing their own collaborations and, once a research proposal is developed, UK and Brazilian applicants must apply jointly for funding. Applicants are responsible for ensuring they follow the correct procedure for both UK and Brazilian funders.
For administrative purposes, all projects will have a Principal Investigator (PI) based at a UK Research Organisation (RO) and a Principal Investigator based at a Brazilian RO.
Grants can be up to 3 years in duration and must start by 1 April 2018 and the end date of the proposed research should be no later than 31 March 2021.
MRC will provide funding for the UK-based applicants under standard arrangements and at 80% FEC. Respective FAP’s will provide funding for the Brazilian applicants according to the usual funding guidelines of each FAP. The size of the grants will vary according to the needs of the research project.
As the UK contribution will be provided by the MRC’s Newton Fund allocation, the research proposed must meet Official Development Assistance (ODA) requirements and be specifically relevant to the Brazilian population. Funding will be awarded in a manner that fits with ODA guidelines. All applications under this call must therefore be compliant with these guidelines to be deemed eligible.
For further information on ODA please visit: www.newtonfund.ac.uk/about/what-is-oda
2. Application Process (índice)
2.1 Application and review process (índice)
1. Closing date for proposals on Thursday 26 September 2017.
2. Joint peer review process including UK and Brazilian academic reviews.
3. Joint panel meetings of UK and Brazilian academic experts, January 2018.
2.2 UK applicants (índice)
The following documents should be submitted via Je-S by 16:00 BST, Thursday 26 September 2017 (https://je-s.rcuk.ac.uk)
-
A completed Je-S form
All UK and Brazilian investigators MUST be included. This form reflects the UK costs, so while the Brazilian investigators should be included, hours charged for the Brazilian investigators should be 0. A breakdown and justification of Brazilian costs should be included in the Justification of resources
-
A cover letter (optional)
-
A jointly prepared Case for Support (see section 3). An additional one-page annex (if required) can be attached to the Case for Support detailing the methodology and experimental design aspects. The use of this annex is strongly advised where the proposal included the use of animals and/or human participants, or where the methodology /experimental design proposed is practically novel. Please see section 2.2.3.4 in the MRC Guidance for applicants
-
Brazilian costs pro-forma
-
Justification of Resources for the total costs requested for the project (both UK and Brazilian costs should be fully justified)
-
Pathways to Impact - please see section 4
-
CV’s + Publications (uploaded individually) for each of the UK and Brazilian partners named on the grant
-
Data Management Plan
-
Letters of Support
-
From both the UK and Brazilian Research Organisations demonstrating support for the proposed project.
-
Where the Brazilian partner or third party (any organisation other than the UK RO) is responsible for recruitment of people as research participants.
-
From any project partner where an in-kind payment is being contributed.
-
Most of the requirements are the same as for a standard MRC application. The MRC Guidance for Applicants and Award Holders can be found on the MRC website: www.mrc.ac.uk/funding/guidance-for-applicants.
All attachments should be completed in 11 point Arial typeface, with a minimum of 2cm margins. Applications will not be accepted where smaller or narrow typefaces have been used.
2.3 Brazilian applicants (índice)
You must submit a combined PDF of the above documents (identical) to CONFAP (and FAPESP where relevant – please see full FAPESP guidelines at www.fapesp.br/11089):
-
Case for Support
-
Brazilian costs pro-forma
-
Justification of Resources
-
Pathways to Impact
2.4 Page Lengths (índice)
(A4 size)
|
MRC-CONFAP – Health Systems Research |
Maximum No of Pages |
|
Case for Support |
6 (plus 1 page for references) |
|
Justification of Resources |
3 |
|
CV’s |
2 |
|
Publications |
1 |
|
Letters of Support |
2 |
|
Pathways to Impact |
2 |
|
Data Management Plan |
3 |
|
Covering Letter |
2 (2 per letter) |
3. Additional document information (índice)
3.1 Case for Support (índice)
The case for support should not exceed 6 sides of A4. You may include one additional side for references, so the document can be 7 sides in total. Your Case for Support must be attached to your Je-S online application as a PDF.
Section 1: Identification of the Team Members
Please complete the below table for all Brazilian and UK applicants
|
Name |
Institution / Department (it should include the address) |
Brazilian State Funding Agency |
Contac information (telephone and e-mail) |
Area of Research |
Link of CV available at Plataforma Lattes |
Role in the Research (PI/Co-I etc) |
|
|
1 |
|||||||
|
2 |
|||||||
|
3 |
|||||||
|
4 |
It is required to include the information of all team members
Section 2: Information about the research proposal (6 pages)
Please use the following headings and the guidance below.
3.1.1 Research project summary
3.1.2 Health Systems relevance and importance
3.1.3 Theoretical Framework
3.1.4 Project and methodology description
3.1.5 Scientific potential and expected outcomes
3.1.6 Research project team, partnership and capacity building
3.1.7 Data management plan
3.1.8 ODA Relevance
3.1.9 Ethics
3.1.1 Research Project summary information
-
Full title of the project (no more than 150 characters)
-
Duration in months and proposed timeline
-
Total amount requested from the MRC and the total budget of the project (UK and Brazil)
-
Principal health systems research question to be addressed
|
Funding Organisation (MRC/FAP) |
UK funding requested at 80% FEC (as indicated in the Je-S proposal form) |
Brazilian funding requested from this scheme (GBP) |
Total funding requested (GBP) |
3.1.2 Health Systems relevance and importance: why is this research needed now and in this proposed location?
-
What evidence is there that the answer to your research question is needed and wanted by relevant users, for instance, policy-makers and research users?
-
Please discuss issues such as burden of disease and priority for local and national health systems and how this research will strengthen local health systems.
-
Proposals must outline why the research is important to this particular context and ensure that the proposal clearly addresses a health system research question rather than, for example, only evaluating the effects of a specific health service intervention.
-
You must demonstrate knowledge of relevant health systems empirical literature and propose how this research may contribute to this literature.
3.1.3 Theoretical Framework
Please describe how your research questions and methodologies are embedded within a suitable theoretical framework with reference to relevant scholarly literature. This framework could draw from any of the appropriate disciplines (economics, sociology, anthropology, political science etc.) and should demonstrate an understanding of theories and concepts that are relevant to your research focus. Where appropriate, proposals should also outline what contribution this research will make to an advance in theoretical knowledge.
3.1.4 Project and methodology description
Please describe your proposed research project, ensuring that you cover the following points:
-
Where will the research take place?
-
Who will the research participants be and why?
-
What pilot or preliminary information do you have available to help the panel assess the feasibility of the proposed study? Costly and complex studies are welcome but applicants should ensure that research questions remain tight and focused and that a potential Panel would be confident any large study could be managed effectively in the field.
-
If you are testing delivery of an intervention, please be clear about what that intervention will consist of and why. How would delivery of the intervention relate to the broader health system and contribute to health systems strengthening.
-
Consider how the findings from the proposed study, especially if it has a single disease focus, may inform work on other related conditions or diseases.
-
If the research involves data collection or acquisition you must demonstrate that you have carried out a datasets review, and explicitly state why currently available datasets are inadequate for the proposed research.
-
What is the proposed timeline?
-
How will you evaluate the outcomes of the study?
Methodology
Give details of the methodological approaches, study design and techniques that will be used. Particular care should be taken to explain any innovation in the methodology or where you intend to develop new methods. The use of qualitative methodology is welcomed where appropriate but researchers should take care to ensure that this is sufficiently detailed and justified. Applicants must ensure that the proposed methodology is appropriate to addressing their research questions and that any countries of focus are well justified. The panel will take a broad view of appropriate methodologies proposed to conduct systems based research.
3.1.5 Research project team, partnership and capacity building
How does the team of investigators incorporate the range of discipline and experience necessary to carry out the study? To what extent is this application led and/or informed by Brazilian researchers? You should explain and justify how this research project will build a research network between UK and Brazilian researchers and institutions. Please ensure budget breakdowns between high income and Brazilian researchers are appropriate with the aims of the scheme and ODA funding. Please outline any plans for capacity building including:
-
Co-design of research;
-
Opportunities for researchers and junior researchers to undertake training and author/co-author journal and conference papers.
Successful proposals will also demonstrate a strong understanding of the local research context and ensure the research programme does not undermine local research capacity.
3.1.6 Data management plan
Does the data management plan indicate whether the applicants have (or are likely to have) a sound plan for managing the research data funded through the award, taking account the types, scale and complexity of data being (or to be) managed o the likely long-term value for further research including by sharing data.
3.1.7 ODA Relevance
Please provide a short paragraph to explain how this research is compliant with the Newton Funds ODA requirements: www.newtonfund.ac.uk/about/what-is-oda
3.1.8 Ethics
It is essential that applicants describe the ethical considerations that have informed the proposed research. Details of the ethical review and research governance arrangements that would apply to the proposal must be described.
3.2 Pathways to impact (índice)
It should be clear to reviewers what the likelihood is of the results of the research being implemented into policy and practice and how you have shaped your proposal to achieve this. For instance, which partner organisations, such as relevant Ministries of Health, have been included in the proposal to ensure relevance and future sustainability of the research outcomes?
-
What changes might be implemented as a result of the study? Please identify practical solutions to implementing health care improvements for vulnerable communities in Brazil.
-
Who will make those changes happen and how?
-
Might the results be generalizable beyond the immediate research setting?
-
Please describe how this research will engage with in-country actors such as academic and non-academic stakeholders and policy makers. Research engagement strategies should reflect stakeholder priorities and any interventions that require government buy-in should demonstrate engagement with public sector actors.
4. Budget (índice)
UK-based research costs will be funded at 80% of the Full Economic Cost. It is the responsibility of the Brazilian and UK PIs to ensure the conditions of their respective funder is understood.
Full Economic Costing (FEC)
Please see section 5. Resources – Full Economic Costing in the MRC Guidance for applicants for information on FEC.
All the UK and Brazilian PI(s)/Co-I(s) must be inputted onto the Je-S form. However, any costs for Brazilian PI(s)/Co-I(s) (unless agreed) must be inputted with hours and charged as £0. A break down and justification of Brazilian costs should be included in the Justification of Resources template.
Funding available
|
MRC funding* |
Brazilian funding |
|
|
Research costs: |
|
|
|
Equipment below £10k |
Yes |
|
|
Equipment above £10k |
No |
|
|
Consumables |
Yes |
|
|
Research studentships/assistants |
Yes |
|
|
Studentships (degree programmes) |
No |
|
|
Travel and subsistence for exchange/mobility activities |
Yes |
|
|
Cost of workshops, meetings etc. |
Yes |
*MRC funding will be provided to the UK HEI but can be spent on activities in Brazil which are outside of the funding available from the Brazilian funders, when identified and justified in the proposal. This must be agreed in advance of submission with the funders.
Equipment:
Capital costs above £10,000 cannot be funded via the Newton Fund and therefore any capital costs requested will not be accepted.
Costs for “small equipment” that are considered consumables are accepted.
5. Joint electronic System (Je-S) (índice)
5.1 Creating a Je-S account (índice)
Please login to your Je-S account via https://jes.rcuk.ac.uk/JeS2WebLoginSite/Logout.aspx, using the username and password you have chosen (if you do not have a Je-S account, or have forgotten your password, please see the guidance provided further below).
-
Select ‘Documents’ from left hand menu list from your Je-S account home page
-
Select ‘New Document’ from within the Functions/create section of your documents page
Creating your Je-S application:
All PIs and CO-Is involved in a grant project will need to be registered on Je-S. Please read on for information about setting up a Je-S account.
The below ‘Call/Type/Mode’ can only be selected when the call opening date has been reached (until the advertised closing date Thursday, 26 September 2017).
All MRC funding calls close at 4pm (16:00 GMT/BST), on the advertised closing date Thursday, 26 September 2017.
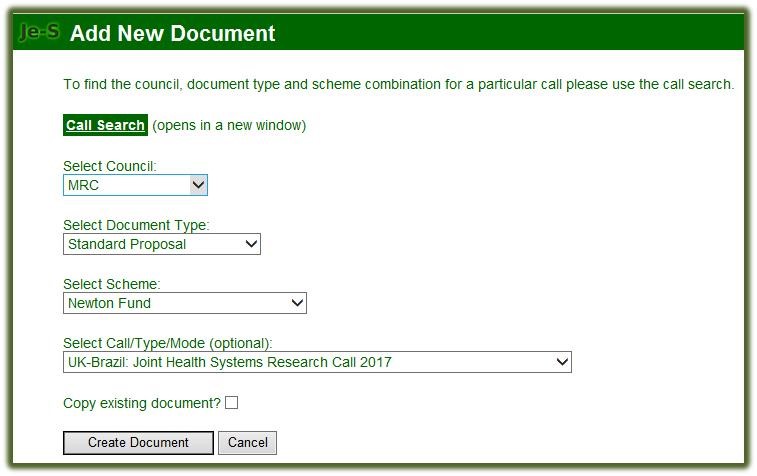
-
Select Council: MRC
-
Select Document Type: Standard Proposal
-
Select Scheme: Newton Fund
-
Select Call/Type/Mode (optional): UK-Brazil: Joint Health Systems Research Call 2017
-
Select ‘Create Document’ option
New Je-S Users: In order to gain access to the Je-S System, Create an Account.
Je-S users having problems successfully completing login to their Je-S account: Retrieve User Name / Password.
Please telephone Je-S Helpdesk 01793 444164 should you require any assistance with the Je-S System
Project Details: Please allow a latest start date of 1 April 2018 (dates selected after this date should fail validation), which it does as per below:

New Je-S Users: In order to gain access to the Je-S System, Create an Account.
Je-S users having problems successfully completing login to their Je-S account: Retrieve User Name / Password.
Please telephone Je-S Helpdesk 01793 444164 should you require any assistance with the Je-S System.
5.2 Guidance for Overseas Organisations to be registered on J-S (índice)
All proposals submitted to this scheme are required to include investigators based in the low or middle income country where the research will take place.
All overseas researchers, investigators and their associated organisations that are included on your proposal, either as lead investigator or co-investigator, must be registered on the Je-S system.
You need to be aware that all Overseas Research Organisations/Institutes and individual applicants (Principal and Co-Investigators), are required to be registered on the Je-S system.
Therefore, both UK organisations and overseas organisations are encouraged to contact us at least two weeks before the call deadline of the 26 September 2017, so we can ensure that the overseas organisation (either Lead or Non-lead), has been correctly added to the Je-S System. Any delays could mean the proposal being rejected because of late submission.
Please email the below detailed information to JeSHelp@rcuk.ac.uk. The helpdesk will then check the Je-S database and advise you accordingly what your next steps should be to Je-S register your organisation and Investigators.
Please provide the Helpdesk with the following information:
1) The name of the overseas organisation/institute (in full).
2) Contact name and email address at overseas organisation/institute.
3) The full postal address of the overseas institution.
4) List the departments associated with the organisation. If the department structure is not known or there no department structure please state the department as ‘Research’.
5) Please include a web link/URL for the overseas organisation/institution (if available).
When the overseas organisation has been added to the Je-S database, this will then enable the overseas applicants (PI and/or Co-Is), to create their Je-S accounts.
Please note that each individual applicant (PI or Co-I), is required to create their own Je-S account, once their organisation has been added to the Je-s system.
Important: Please allow sufficient time (before the call closes), E.G. 3-4 hours, to allow them time to login to their Je-S account, allocate the document to their own account and then complete the submission process. Should you require any assistance with the submission process, please contact the Je-S Helpdesk.
6. Assessment Process and Criteria (índice)
The assessment panel for this scheme will consider whether applications are of world-class standard (being intellectually innovative, well-focused and methodologically sound), and whether the research has the potential to make real improvement to health in low and middle-income countries.
Peer reviewers and Panel members will be asked to comment on the following criteria in assessing the outline proposals:
Research Quality
-
Scientific Rationale: novelty, importance and timeliness of the research and whether this is likely to lead to new understanding;
-
Has the proposal outlined a need and justification for the proposed research area and situated the work within an existing body of literature?
-
Has the proposal demonstrated engagement with relevant theoretical frameworks?
Impact
-
Will this research generate evidence on how to strengthen and improve health systems for people living in Brazil? Have practical solutions to implementing health care improvements for vulnerable communities in Brazil been identified?
-
Has this research used a health systems approach to inform the delivery of evidence-based interventions or structural changes? Proposals must demonstrate how interventions relate to and affect wider elements of a health system such as governance, financing, health workforce, information systems, service delivery etc.
-
Will this research provide evidence that is of direct relevance to decision makers and practitioners in the field?
-
Has the proposal identified potential barriers to uptake of the research outcomes in the setting and proposed plans to overcome these?
-
Has this proposal identified key factors relevant to the potential scalability of the research?
-
What is the likelihood that the findings will be taken up and implemented? Is there a coherent plan for research uptake? Can the approach be scaled up; is it cost effective?
Research Management, People and Partnership
-
The suitability of the investigator group including track record(s) of the individuals in their field(s) and whether they are best-placed to deliver the proposed research.
-
How have team members from different disciplines been included and how has their variety of input been embedded in the approach to research? (e.g. are methodological, social, health systems, economics, cultural issues covered?)
-
The management strategy proposed, including equitable access to any shared resources and sufficient capability and time commitments of senior staff to steer and oversee the research.
-
Links with relevant local research/health institutions and involvement of investigators from Brazil that will build a strong and important partnership.
-
Have opportunities for research capacity building been embedded into research plans?
Methodology
-
The feasibility of experimental plans, statistics, methodology and design, including provision of sample size calculations, strategies to avoid bias, and preliminary data where appropriate;
-
Is the design of the study appropriate to answer the question?
-
Is the timeline is realistic and achievable?
-
Has the methodology been underpinned by a relevant theoretical or conceptual framework.
-
Have major scientific, technical or organisational challenges been identified, and will they be tackled well?
Ethics
Proposals must engage with ethical and/or research governance issues, including whether proposed research is ethically acceptable and the appropriateness of ethical review and research governance arrangements.
Data Management Plan
Is there is a sound plan for managing the research data, taking into account the types, scale and complexity of data being (or to be) managed and also the likely long-term value for further research including by sharing data?
Resources Requested
Does the proposed research demonstrate good value for money?
Are the funds requested essential and justified by the importance and scientific potential of the research?
Official Development Assistance (ODA) compliance
Research proposals must be in line with the RCUK ODA compliance. www.rcuk.ac.uk/documents/international/gcrfodaguidance-pdf
The primary focus of research should be the long term sustainable growth of Brazil.
7. Agreements (índice)
7.1 Collaboration Agreement (índice)
As the research projects will be carried out by multiple research organisations and project partners, the basis of collaboration between the organisations and project partners, including ownership of intellectual property (IP) generated during the project and rights to exploitation, and costs of IP management [this is not an eligible cost to MRC], is expected to be set out in a formal collaboration agreement between the research organisations involved. It is the responsibility of the research organisations to put such an agreement in place before the research begins. The terms of collaboration shall not conflict with MRC and the respective FAPs terms and conditions.
Arrangements for collaboration and/or exploitation must not prevent the future progression of academic research and the dissemination of research results in accordance with academic custom and practise and the requirements of the funding bodies. A temporary delay in publication is acceptable in order to allow commercial and collaborative arrangements to be established.
Details of key issues included in the Collaboration Agreement, for example management of IP, should be detailed in the ‘consideration of ethical, governance and IP issues around the project’ section of the Case for Support.
7.2 Intellectual Property (índice)
Ownership of intellectual property (IP) generated during the project and rights to exploitation, as well as any costs regarding management of IP, are expected to be agreed between the collaborating research organisations before the research begins. Details of this agreement should be included in the Collaboration Agreement (as above).
Agreements must not conflict with MRC and the respective FAPs terms and conditions. Any agreements in place between a research organisation and their respective funding organisation must be adhered to, including the sharing of IP costs or benefits. Any IP sharing agreements in place between a research organisation and their national funding body would be expected to apply only to the IP share of that research organisation.
7.3 Material Transfer Agreements (índice)
Collection and exchange of material may occur between collaborating institutions, as necessary, in strict compliance with the legislation in effect in both countries.
7.4 Ethics (índice)
Any research involving humans/human tissue and/or animals must comply with legislation in both the UK and Brazil, and must also comply with relevant policies and guidance of MRC and the respective FAPs.
It is the absolute responsibility of the PIs and the ROs to ensure that appropriate ethical approval is granted and adhered to, and that no research requiring ethical approval is initiated until it has been granted.
The Ethical Information sub-sections in the Je-S proposal form should be completed to give details of any human participation, research using animals, genetic and biological risk, and ethical committee approvals required. Section 5 of the MRC Guidance for applicants has recently been updated to reflect amendments to this section of the Je-S form.
Applicants must be clear in their applications in which country the proposed research involving humans and/or animals will take place and must fully complete the Ethical Information section for research taking place in either country.
MRC Ethics guidance
Applicants must comply with all of the MRC’s relevant policies and guidance regarding the use of humans/human tissue and/or animals in research.
Approval(s) for the research detailed in an MRC grant proposal must be granted by the appropriate bodies before any work can commence. Institutions, applicants and grant holders have absolute responsibility for ensuring that the necessary approvals are granted for the research considered by MRC and the respective FAPs.
The Principal Investigator/ Research Organisation must be prepared to furnish the MRC with a copy of the ethical approval, and any correspondence with the committees, if requested by the Council. The principal investigator must notify the MRC if a regulator or a research ethics committee requires amendments that substantially affect the research question, methodology or costs to the extent that the project is no longer the same as that approved for funding by the MRC.
7.5 Humans/Human Tissue (índice)
MRC guidance
Applicants must comply with relevant MRC policies and guidance (section 5 of the MRC Guidance for applicants)
In particular, applicants should be aware of the following guidance/requirements:
MRC current policy for research involving humans to take place overseas www.mrc.ac.uk/news-events/publications/research-involving-human-participants-in-developing-societies is that for research to be undertaken internationally, both local and UK ethical approval is required.
For clinical studies involving human participants and/or patients in the UK or overseas, appropriate consent must be obtained.
Where the Brazilian partner or another third party (ANY organisation other than the UK RO) is responsible for recruitment of people as research participants and/or providing human tissue, details should be included in the case for support and a letter of support MUST be attached to the application. The letter of support should be titled Human participation and include confirmation of the following:
-
That the international partner has agreed to recruit the participants/provide tissue
-
That what is being supplied is suitable for the research being undertaken
-
That the quantity of tissue (where relevant) being supplied is suitable, but not excessive for achieving meaningful results
The letter of support must be an integral part of the application (as an attachment) and must focus on the proposal it accompanies.
8. Terms and Conditions (índice)
MRC www.mrc.ac.uk/documents/pdf/mrc-additional-terms-and-conditions
RCUK www.rcuk.ac.uk/funding/grantstcs
Recipients of grants from the MRC are required to adhere to the MRC terms and conditions www.mrc.ac.uk/documents/pdf/mrc-additional-terms-and-conditions
Newton Fund terms and conditions are provided below:
ODA compliance
The Newton Fund is part of the UK’s Official Development Assistance (ODA). Its aim is to develop science and innovation partnerships that promote the economic development and welfare of developing countries. The investigators must ensure the research part of this grant remains compliant with ODA rules and regulations as set out under the Newton Fund programme. In the event that the research does not remain compliant with ODA rules and regulations Medical Research Council reserve the right to terminate the award. And recoup any funds as appropriate.
Acknowledgements and reporting
Investigators must acknowledge the Newton Fund and the Medical Research Council in any publications, web pages or events associated with this grant.
Investigators must assist the Medical Research Council with any additional reporting requirements requested by the Department for Business, Energy and Industrial Strategy or any other government department.
Starting Procedures
This grant should start by 1 April 2018. The start of the grant may NOT be delayed beyond this date.
Please note that due to the fixed start date, the normal three months start period rules outlined in the RCUK Terms and Conditions RGC4, does not apply to this project.
Ethical Requirements
It is the responsibility of the Principal Investigator and the Research Organisation to ensure that appropriate ethical approval is granted for this study and adhered to, and that no research requiring ethical approval is initiated until it has been granted.
MRC current policy for research involving humans www.mrc.ac.uk/news-events/publications/research-involving-human-participants-in-developing-societies is that for research to be undertaken overseas, both local and UK ethical approval is required.
For clinical studies involving human participants and/or patients appropriate consent must be obtained.
For grants that include the use of animals, the guidance www.mrc.ac.uk/news-events/publications/responsibility-in-the-use-of-animals-in-research must be adhered to, and in particular: 'When collaborating with other laboratories, or where animal facilities are provided by third parties, researchers and the local ethics committee in the UK should satisfy themselves that welfare standards consistent with the principals of UK legislation (e.g. the ASPA) and set out in this guidance are applied and maintained.'
The Principal Investigator/Research Organisation must be prepared to furnish the Medical Research Council with a copy of the ethical approval, and any correspondence with the committees, if requested. The Principal Investigator must notify the Medical Research Council if a regulator or a research ethics committee requires amendments that substantially affect the research question, methodology or costs to the extent that the project is no longer the same as that approved for funding.
Government Support
This award is dependent on continuing Government commitment for this initiative and continuing match from (Partner funder). In the event that this support if withdrawn, the Medical Research Council reserve the right to terminate the award.
Requests for extensions to awards
Due to financial restraints of the Newton Fund Programme, grant extensions will only be considered under exceptional circumstances (in line with the Equality Act 2010) and will require the Medical Research Councils’ agreement on a case-by-case basis. The Research Organisation remains responsible for compliance with the terms of the Equality Act 2010 including any subsequent amendments introduced while work is in progress; and for ensuring that the expectations set out in the Medical Research Councils’ statement of expectations for equality and diversity are met.
Transfer of funds to UK and overseas organisations
It is important to highlight that the Research Organisation awarded the grant is responsible for the conduct and administration of the grant during the life time of the award (from award, during the grant and on completion). It is accountable for the effective use of public funds, and must therefore ensure that all grant monies are subject to proper financial management processes. It is the Research Organisation’s responsibility to ensure that, where funds are transferred to other organisations in the UK and abroad, expenditure is subject to robust controls to ensure value for money and propriety and that all costs should be fully vouched and maintained for possible inspection and checks by, or on behalf of, the funding organisation.
This award has therefore been made on the basis that if any funds are transferred to another UK or overseas organisation then the Research Organisation must undertake due diligence checks to ensure that the funding will be appropriately used (as set out above). The Research Organisation may be asked to provide evidence that where funds have been transferred they have undertaken appropriate due diligence to ensure that any risks are recognised, understood and treated as necessary. The Research Organisation may be asked to provide additional information on how the due diligence checks were carried out.
Please refer to the Medical Research Council for any specific guidance.
Collaboration agreement
A Collaboration Agreement is required for this project. This must be in place within six months of the start of the project.
As the grant is associated with more than one research organisation the basis of collaboration between the organisations, including the allocation of resources throughout the project and ownership of intellectual property and rights to exploitation is required to be set out in the formal collaboration agreement. It is the responsibility of the lead Research Organisation to put such an agreement in place within six months of the start of the project. The terms of collaboration agreements must not conflict with the Medical Research Councils' terms and conditions.
Annex 1 (índice)
Additional questions on the use of rodents overseas
The expectations of the Research Councils for the use animals in research are set out in the document ‘Responsibility in the Use of Animals in Bioscience Research’. Compliance with the principles in this document is a condition of receiving funding.
Please confirm the following: (tick box – yes/no)
|
1. The enclosure sizes and space allocations meet or exceed those in Annex VII to Directive 2010/63/EU (Tables 1.1 to 1.5) |
|
|
2. The rodents are provided with: a) substrate/bedding on a solid floor; b) a shelter and/or nesting material for refuge and to help regulate body temperature and light exposure; c) chew blocks or other gnawing material. |
|
|
3. The rodents are housed socially. Exceptions to this must be justified below. |
|
|
4. Appropriate, contemporary anaesthesia and/or analgesia is provided to minimise pain and distress. Any withholding of pain relief during painful procedures must be justified below. |
|
|
5. Surgery is performed using aseptic technique, the least invasive surgical approaches, and appropriate perioperative care (pre-operative medications, hypothermic prevention, ophthalmic protection, nursing care where required). |
|
|
6. Toe clipping and/or tail biopsy are not used for identification or genotyping purposes. |
|
|
7. Where genotypes are known to be harmful, animals of that type are not produced unless required scientifically (e.g. if homozygous null is harmful and heterozygotes are desired, then heterozygous is crossed with wild type, not another heterozygous animal). |
|
|
8. Where new GA strains are being generated, best knowledge will be applied to predict potential harmful outcomes and the animals will be monitored closely for emerging phenotypes. |
|
|
9. The rodents are monitored with a frequency appropriate to keep pain and distress to a minimum, using appropriate, tailored welfare indicators and score sheets. |
|
|
10. Humane endpoints have been established for each experiment with the potential to cause moderate or severe harm, after consultation with the veterinarian and animal care staff, and implementation of these is recorded during the experiment. (Note the humane endpoint criteria may be requested by the Research Councils). |
|
|
11. The methods of humane killing are those recommended by the AVMA (2013) or permitted under Directive 2010/63/EU. |
Where there are deviations from the above, please explain below: (free text; one side of A4)
